Uptake of herpes zoster vaccine linked to supply
Strong uptake led to short supply for the shingles vaccine. Learn how to educate patients and integrate best practices into your clinical routine.
Shingrix, a newly approved subunit vaccine for herpes zoster manufactured by GlaxoSmithKline (GSK), received the stamp of approval in rapid succession from both the FDA and the Advisory Committee on Immunization Practices (ACIP) in fall 2017. In clinical trials, Shingrix had an overall efficacy against shingles of 97.2% in adults ages 50 years and older and maintained protection rates of 84.7% or higher for the following three years in adults ages 70 years and older.
The previously available live attenuated herpes zoster vaccine, Zostavax, which is manufactured by Merck and was approved in 2006, had 51% efficacy against shingles and 66.5% efficacy against postherpetic neuralgia over a 3.12-year period. Studies of Zostavax found that effectiveness decreased substantially after the first year and was below 35% by six years after vaccination.
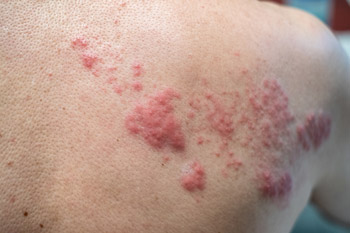
Favorable buzz regarding Shingrix's efficacy and quick coverage by most types of insurance have led to an enthusiastic reception by patients, said Jack Ende, MD, MACP, a general internist at Penn Medicine in Philadelphia. “I have a lot of patients asking me for it, asking me about it,” said Dr. Ende. Due in part to that enthusiasm, the vaccine has been in short supply.
“I think it caught the manufacturer by surprise, what a strong uptake there was,” Dr. Ende said. “They just can't keep up with the demand.”
ACIP approval
Soon after Shingrix's FDA approval, on Oct. 25, 2017, the CDC's Advisory Committee on Immunization Practices (ACIP) recommended the new vaccine over Zostavax for the prevention of herpes zoster and related complications in immunocompetent adults ages 50 years and older. The ACIP also recommended Shingrix for immunocompetent adults who had previously received Zostavax.
The ACIP's recommendations, which were published Jan. 26, 2018, in Morbidity and Mortality Weekly Report (MMWR), added an additional 62 million people to the pool of potential Shingrix recipients, for a total of 115 million, according to GSK.
“Once it got the ACIP seal of approval, which does not usually happen so quickly for a vaccine, that really got people's attention,” said John O’Neill Jr., DO, MACP, an internist in Middletown, Del., and former Chair of ACP's Immunization Technical Advisory Committee.
Under the Affordable Care Act, all Health Insurance Marketplace plans and most private health insurance plans were required to cover Shingrix within a year of publication of the ACIP recommendations in MMWR. According to GSK, by October 2018, the vaccine was covered for more than 95% of U.S. commercial medical benefit enrollees and 100% of Medicare Part D enrollees.
The quick action by the ACIP and the ensuing embrace by insurers compressed what is typically a nine- to 12-month cycle for the uptake of new vaccines into four months, said Len Friedland, MD, vice president and director of scientific affairs and public health vaccines at GSK. “This has never been seen before for an adult vaccine.”
That demand and interest are also due in part to the public's increased knowledge of shingles, said Carolyn Bridges, MD, FACP, a consultant for the Immunization Action Coalition who served as past director of adult immunization at the CDC until retiring two years ago.
“There's greater awareness of shingles. Everybody knows what that looks like and how debilitating it can be,” said Dr. Bridges, who is the current Chair of ACP's Immunization Committee. Regarding insurance, she noted that although Medicare Part D plans cover the new vaccine, the out-of-pocket costs to patients may be substantial.
Tradeoffs
The efficiency and longevity of the new vaccine come with some tradeoffs. In phase III clinical trials, about 85% of patients who received Shingrix reported injection-site and systemic reactions, and 17% experienced grade 3 reactions. Those rates are considerably higher than those seen with Zostavax. According to data presented by ACIP in February based on 8.59 million doses administered between October 2017 and December 2018, reports of adverse events were “generally consistent with the safety profile observed in prelicensure clinical trials.”
Dr. Ende said he has been called about side effects for a few patients, for example, a fever for a day or two after getting the vaccine. David Reuben, MD, FACP, chief of geriatrics at the University of California, Los Angeles, David Geffen School of Medicine, said that in his experience with a geriatric population, the side effects of the new vaccine are considerable.
“A fairly substantial number of people who take it get very sore arms. Then there are some people who have a myalgia and flu-like kind of feeling … for a few days.” He added that some of his patients who had previously gotten Zostavax are holding off on Shingrix because of what they have heard about side effects.
Dr. Bridges said that because of the known side effects of Shingrix, educating patients beforehand is key. Just the simple act of scheduling can make a difference to a patient's experience. “Make sure they don't plan it for the same weekend that their grandchild is getting married or they have a graduation,” she said. When she got the vaccine, Dr. Bridges said, she did it on a Friday so she'd have the weekend to deal with any side effects that might arise.
It's also important to remind patients of the relatively short-lived side effects of the vaccine versus risking a case of shingles—two or three days of discomfort, fatigue, or fever versus several weeks or months of pain, Dr. Bridges said. She added that the general ACIP recommendations allow for giving inactivated vaccines together, so she does not see a problem with physicians administering Shingrix with other inactivated vaccines if scheduling is a concern.
Dr. O’Neill believes, however, that although some patients do prefer getting more than one vaccine at a time, the best approach is to administer Shingrix solo. “That way, the patients are just dealing with the side effects from the Shingrix vaccine alone, and that makes it easier to keep track of where the side effects are coming from,” he said.
Dual doses
Unlike Zostavax, which required only one dose, Shingrix is given in two doses, the second two to six months after the first. According to data presented in February by the ACIP, approximately 1.5 million Medicare beneficiaries in six states received the vaccine between October 2017 and December 2018. Approximately 77% of the beneficiaries received their second dose within six months of the first, and by nine months, approximately 85% had received the second dose.
GSK's Dr. Friedland said their internal numbers are similar. As of June, their data showed that 75% of patients who received the first dose had gotten the second dose within six months. He added that GSK did not have data out to nine months because that is not part of its dosing regimen.
In guidance published Jan. 26, 2018, in MMWR, the CDC stated that if more than six months have elapsed since the first Shingrix dose, there is no need to restart the vaccine series. “However, the efficacy of alternative dosing regimens has not been evaluated, data regarding the safety of alternative regimens are limited, and individuals might remain at risk for herpes zoster” until they get the second dose of vaccine, the report said.
Where's the vaccine?
Due to the demand for Shingrix, supply has been spotty. At a session at ACP's Internal Medicine Meeting 2019 in New Orleans in April, for which Dr. O’Neill served as faculty, physicians reported problems getting the vaccine. For example, Dr. O’Neill said, one physician stated that one of his patients got a first dose at a pharmacy but couldn't get the second dose six months later because it was on back order, and another physician reported simply being unable to obtain the vaccine for the office.
In Los Angeles, Dr. Reuben said, the situation has been particularly challenging. “It just disappears from the entire city, that's what we've found, and then it reappears two or three months later,” he said.
As of March, GSK had distributed up to 12 million doses of the vaccine, Dr. Friedland said, and in April it announced a $100 million investment to expand production capacity at an existing plant in Montana where components for Shingrix and other vaccines are manufactured. GSK also recently got approval from the FDA to bring a vaccine plant in France online.
“There was no precedent in the past to predict how this would play out,” Dr. Friedland said. “So, we're working hard to solve the good situation that we're in, but we recognize that it's challenging, and it's frustrating.”





